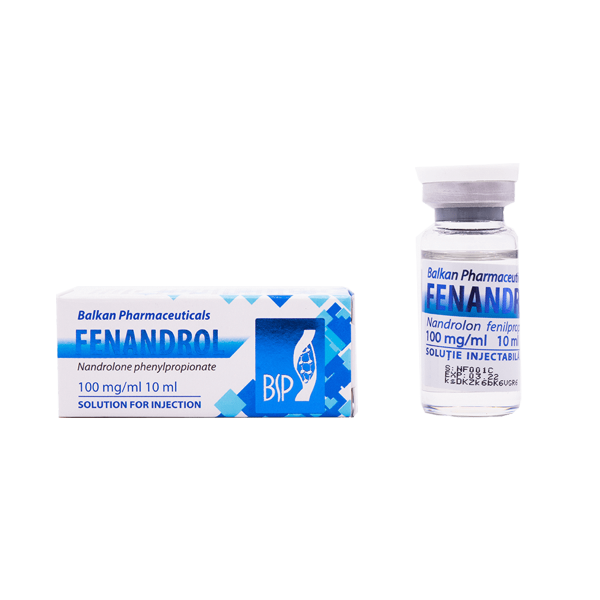
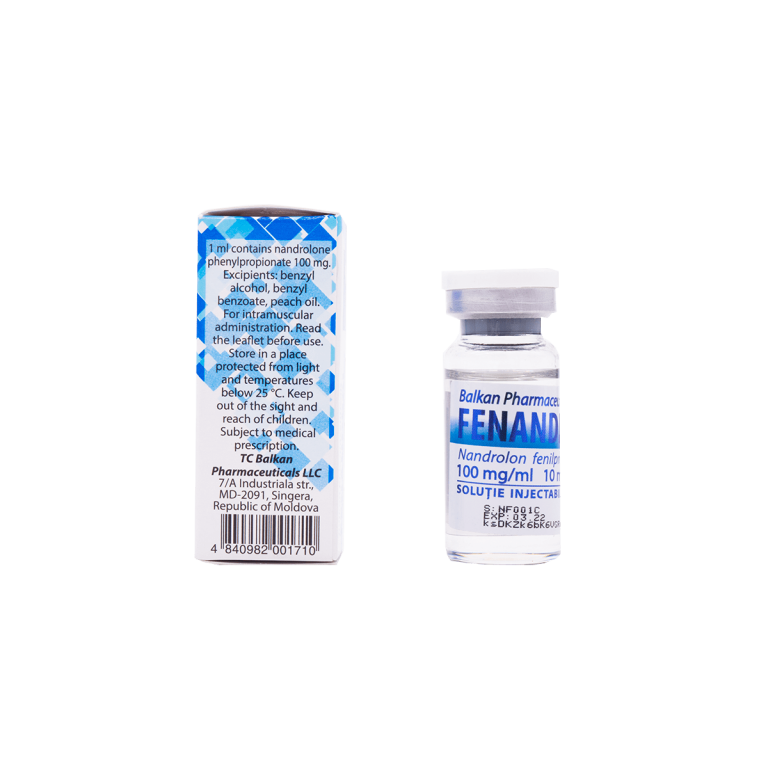
BP FENANDROL 10ml
$50.00 Original price was: $50.00.$45.00Current price is: $45.00.
Package:1 vial (100 mg/ml 10ml)
Active Substance: Nandrolone Phenylpropionate
Indication:
In men, Fenandrol from Balkan Pharmaceuticals, also known as Nandrolone Phenylpropionate is administered to treat androgenic deficit after castration, eunuchoidism, hypopituitarism, hormone-induced impotence, and symptoms of male climacteric (decreased libido and physical and intellectual activity). It is also used for treating certain forms of infertility with impaired spermatogenesis and osteoporosis caused by androgen insufficiency. In women, Nandrolone-F is utilized to manage high levels of estrogen, uterine myoma, endometriosis, breast cancer, and osteoporosis.
Contra-indications:
Nandrolone-F is not indicated for use in individuals with hypersensitivity to the drug, prostate and breast carcinoma, prostate hyperplasia with urination disorders, nephrosis or nephrotic phase of nephritis, edema, hypercalcemia, liver function disturbances, diabetes, heart failure or coronary disease, myocardial infarction, and atherosclerosis in elderly men. It is also contraindicated during pregnancy and lactation.
Administration:
Nandrolone Phenylpropionate is administered by deep intramuscular injection. The dosage is determined individually based on the indication and the patient’s response.
Typically, adults receive 1 ml intramuscularly once every 14 days. When a therapeutic effect is achieved, the dosage is adjusted to 1 ml intramuscularly once every 28 days. The duration of treatment is determined on an individual basis.
For male sterility (azoospermia, oligospermia), the dosage is 2 ml once every 2 weeks (treatment is discontinued if painful erections occur).
In breast cancer in women, the dosage is 1-2 ml every 1-2 weeks during the treatment period.
The drug must not be administered intravenously.
Medical action:
Nandrolone-F is composed of Nandrolone Phenylpropionate, a synthetic anabolic steroid derived from testosterone.
Nandrolone Phenylpropionate has strong anabolic and moderately androgenic properties. It promotes protein synthesis, reduces protein catabolism, and enhances nitrogen retention in muscle tissues, leading to increased muscle mass and strength. It also stimulates red blood cell production and improves oxygen transportation in the body, resulting in enhanced endurance and recovery.
In women, Nandrolone Phenylpropionate inhibits pituitary gonadotropic function, ovarian function, and mammary glands, leading to endometrial atrophy. Due to its antagonistic action against estrogen, it is useful in treating uterine myoma, endometriosis, and breast cancer. It also manifests beneficial effects during the climacteric period.
Precautions:
If androgen-dependent adverse reactions occur, it is necessary to discontinue the medication. After the disappearance of adverse reactions, treatment may be resumed at lower doses.
Patients with latent or overt cardiac failure, impaired renal function, hypertension, epilepsy, or migraine (or a history of these conditions) must be under constant supervision due to the possibility of sodium and water retention caused by androgens. Liver function should be monitored during long-term treatment. In patients with breast cancer, hypernephroma, lung cancer, or bone metastases, calcium levels in blood and urine need to be controlled. In prepubertal adolescents, the drug should be administered cautiously to avoid stunted growth and premature puberty.
Side effects:
- Priapism and other symptoms of sexual hyperstimulation (frequent erections);
- Accelerated sexual development in prepubertal teenagers, increased frequency of erections, increased size of sexual organs, and premature epiphyseal closure;
- Impaired spermatogenesis and sperm maturation disorders, oligospermia, and reduced ejaculate volume;
- Prostate abnormalities;
- In women, bleeding from the genital tract, increased libido, and possible virilization symptoms with prolonged administration;
- Hirsutism, gynecomastia;
- Seborrhea, acne, oily skin, hair loss;
- Water and sodium retention, edema;
- Symptoms of hypercalcemia;
- Thrombophlebitis;
- Nausea, cholestatic jaundice, increased liver transaminase levels (which normalize upon discontinuation);
- Headaches, depression, aggression, anxiety, sleep disorders, paresthesias, and possible pain, itching, and redness at the injection site.
Overdosage:
In the event of an overdose, seek immediate medical assistance.
Popular Brands
Popular Categories
-

Winstrol/Stanozolol10 Products
-

Turanabol/4-Chlorodehydromethyltestosterone7 Products
-

Trenbolone Mix8 Products
-

Trenbolone Enanthate9 Products
-

Trenbolone Acetate7 Products
-

Testosterone Propionate8 Products
-

Testosterone Mix14 Products
-

Testosterone Enanthate8 Products
-

Testosterone Cypionate7 Products
-

Primobolan7 Products
-

Parabolan9 Products
-

Oral Steroids48 Products
-

Nandrolone Phenylpropionate7 Products
-

Nandrolone Decanoate6 Products
-

Injectable Steroids137 Products
-

Injectable Mix11 Products
-

Drostanolone Propionate7 Products
-

Drostanolone Enanthate8 Products
-

Dianabol/Methandienone9 Products
-

Boldenone Equipoise9 Products
-

Anavar/Oxandrolone10 Products
-

Anadrol/Oxymetholone5 Products









Reviews
There are no reviews yet.